Covering COVID-19 is a daily Poynter briefing of story ideas about the coronavirus and other timely topics for journalists, written by senior faculty Al Tompkins. Sign up here to have it delivered to your inbox every weekday morning.
Covering COVID-19 is a daily Poynter briefing of story ideas about the coronavirus and other timely topics for journalists, written by senior faculty Al Tompkins. Sign up here to have it delivered to your inbox every weekday morning.
The first coronavirus death in the United States occurred on Feb. 17. Today, Dec. 14, researchers, drug companies, the federal government, a supply chain that runs from the U.S. military to FedEx and UPS, syringe companies, glass vial companies, state coordinators and thousands of people who will never appear on the news have pulled off the medical equivalent of a moon shot. The first COVID-19 vaccinations outside of drug trials begin today in America.
And for all there is to celebrate, Nicholas Kristof puts today in perspective:
If American states were treated as countries, the places with the highest per capita coronavirus death rates would be: Slovenia, South Dakota, North Dakota, Bulgaria, Iowa, Bosnia, Hungary, Croatia, Illinois, North Macedonia, Rhode Island, Nebraska, Kansas, Arkansas, San Marino.
Of note, news broke on Sunday that White House staffers would receive the COVID-19 vaccine ahead of even high-risk front-line workers and seniors in long-term care. The White House is jumping in line even while holding holiday events without masks.
Pharmacists, farmers, store workers, meat plant workers, pilots, teachers, police and prison and jail employees are all pushing to get priority as well.
Pregnant and breastfeeding women may take the vaccine
The weekend’s Food and Drug Administration and Centers for Disease Control and Prevention’s vaccine orders mostly came without any surprises — except this one. The FDA authorized the vaccine for people 16 and older and left open the choice for women who are pregnant and breastfeeding to take the vaccine. It is a surprise because the vaccine has not been tested on pregnant women.
British authorities, who have been administrating the vaccine for a week, did not recommend it for pregnant women but also have noted they have not seen anything that raises concerns about it eventually being given to pregnant women. But British officials say they want more data before they take that step. The British health agency recommends:
if you are pregnant you should not be vaccinated — you can be vaccinated after your pregnancy is over
if you think you may be pregnant you should delay vaccination until you are sure you are not
if you are planning to get pregnant in the next 3 months, you should delay your vaccination
if you know you are not pregnant you can start the two-dose course now and you should avoid getting pregnant until at least 2 months after the second dose
if you have had the first dose and then become pregnant you should delay the second dose until after the pregnancy is over
The guidance from the FDA is different. Dr. Sarah Mbaeyi, an FDA medical officer, said Saturday that any woman considering vaccination should consider the level of COVID-19 in her community, her personal risk of contracting the virus, the risks to her or her fetus of developing the disease, and the vaccine’s known side effects.
CDC will also urge physicians to advise women to take acetaminophen if they develop a fever after vaccination — to protect the developing fetus from fever.
Sandra Fryhofer, MD, representing the American Medical Association, commended the CDC for these recommendations. But she also called on Pfizer, the FDA, and the CDC to make data from the developmental and reproductive toxicity (DART) studies public as soon as possible.
“We really need to put those results on warp speed and get them out there to give our physicians and pregnant women more information,” said Fryhofer, an adjunct associate professor of medicine at Emory University School of Medicine in Atlanta, Georgia.
The American College of Obstetricians and Gynecologists (ACOG) will also soon release guidance for vaccinating pregnant and breastfeeding women, said Linda Eckert, MD, FACOG, an ACOG representative on the panel.
About 8,500 pregnant women have been hospitalized with COVID-19 in the U.S. out of 44,000 known COVID-19 cases involving pregnant women, while 57 pregnant women have died from COVID-19 in the U.S. Those statistics are behind one line of thinking that the virus presents a greater risk than the vaccine. The New York Times reports:
Some experts said the virus itself poses greater risks to pregnant women than the new vaccine, and noted that vaccines have been given to pregnant women for decades and have been overwhelmingly safe.
“This is a really huge step forward in recognizing women’s autonomy to make decisions about their own health care,” said Dr. Emily Miller, an obstetrician at Northwestern University and a member of the Covid-19 task force of the Society for Maternal and Fetal Medicine.
The serious decision about whether to vaccinate pregnant women comes into focus fast when you realize that there are an estimated 330,000 pregnant and breastfeeding health care workers who will be in the priority line to be vaccinated.
Hospitals are admitting fewer COVID-19 patients. Here’s why that might not be good news.
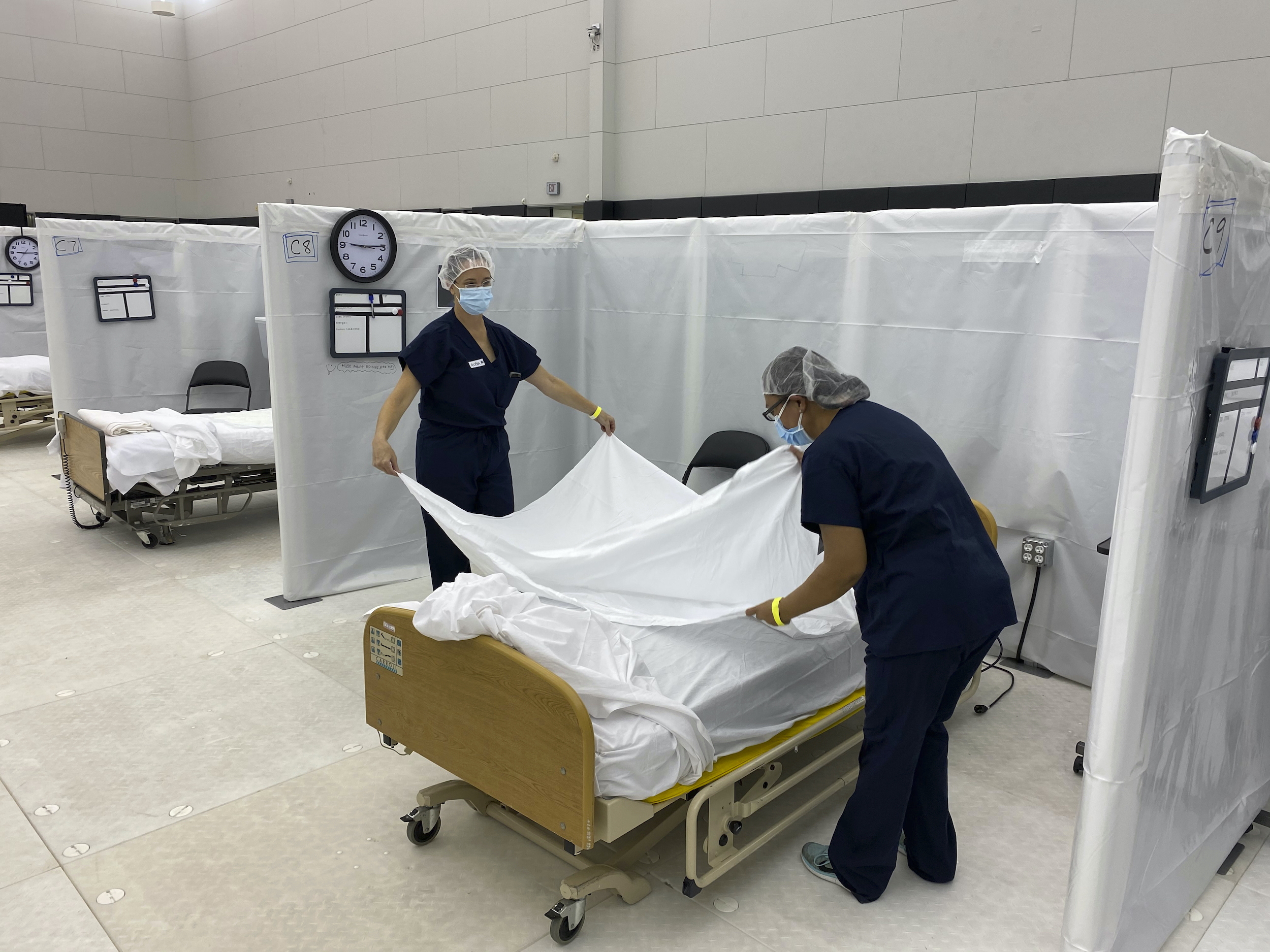
Hospital beds are set up in the practice facility at Sleep Train Arena in Sacramento, Calif., which is ready to receive patients as needed on Dec. 9, 2020. (California OES via AP)
When you go to the hospital, there are several factors that figure in to whether you are admitted. One question is how sick or injured you are. Another factor is how many beds are available.
The mayor of Tulsa, Oklahoma, says hospitals are so full that they are subtly rationing care by raising the bar for who they admit for COVID-19 cases.
Dr. Ashish K. Jha, a professor of health services, policy, and practice and dean of the School of Public Health at Brown University, tweeted that the percentage of people who have COVID-19 and get admitted to a hospital is falling.
There is something funny happening with COVID hospitalizations
Proportion of COVID pts getting hospitalized falling
A lot
Just recently
My theory?
As hospitals fill up, bar for admission rising
A patient who might have been admitted 4 weeks ago may get sent home now
Thread
— Ashish K. Jha, MD, MPH (@ashishkjha) December 1, 2020
“For months,” Jha says, “you could reliably predict new hospitalizations. How? By taking cases seven days prior, multiplying by 3.5% That is 3.5% (1 in 29) of those diagnosed today will be hospitalized about 7 days later.” Jha says that calculation held together right up until Nov. 15, when the percentage dropped to 3%. On Nov. 22, it dropped again to 2.5% and fell to 2.1% on Nov. 29.
He says that when beds were available, COVID-19 cases would be admitted. When hospitals began to fill, there appears to have been a change in the way cases are treated. And, he says, we should be concerned that it is not just COVID-19 cases that are being sent home but other patients with heart problems or infections who may not be admitted today when they would have been a month ago.
MedScape adds that there may be more behind the figures:
Physicians from five hospitals in different parts of the country told Medscape Medical News a different story. Some are not admitting patients with COVID-19 that they otherwise would have in the spring, but doctors say the decisions are based on greater knowledge of the disease, not necessity — yet.
“Right now, I don’t think there’s really anybody that we’re saying, ‘We’re so busy, we don’t have any beds, we normally would admit you but we’re not going to admit you,’” said Robert Saldana, DO, an emergency medicine provider at Houston Methodist Hospital in Houston, Texas. “That is just not happening anywhere that I know of.”
A spokesperson for Nebraska Medicine told Medscape Medical News that the health system had “gotten very close” to changing hospital admissions criteria to accommodate COVID-19 patient volumes, but then hospitalizations have trended downward since mid-November’s count of 1000 statewide.
Want to know what’s in the Pfizer vaccine?

Boxes containing the Pfizer-BioNTech COVID-19 vaccine. (AP Photo/Morry Gash, Pool)
Since there is some concern about whether people who have severe allergic reactions should take the COVID-19 vaccine, it might be useful for those folks to know what is in it. The smart people at MIT deciphered a list of ingredients.
Active Ingredient
- nucleoside-modified messenger RNA (modRNA) encoding the viral spike glycoprotein (S) of SARS-CoV-2
Lipids
- (4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis (ALC-3015)
- (2- hexyldecanoate),2-[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide (ALC-0159)
- 1,2-distearoyl-snglycero-3-phosphocholine (DPSC)
- cholesterol
Salts
- potassium chloride
- monobasic potassium phosphate
- sodium chloride
- basic sodium phosphate dihydrate
Other
- sucrose
If you want to understand what all of that stuff does, go here. They also explain how the technology in this vaccine works. This is important. MIT Technology Review says:
Pfizer makes a point of saying its mixture of lipid nanoparticles and mRNA is “preservative-free.” That’s because a preservative that’s been used in other vaccines, thimerosal (which contains mercury and is there to kill any bacteria that might contaminate a vial), has been at the center of worries around over whether vaccines cause autism. The US Centers for Disease Control says thimerosal is safe; despite that, its use is being phased out. There is no thimerosal — or any other preservative — in the Pfizer vaccine.
Local journalists: Top vaccine experts answer your questions today
At 2 p.m. Eastern today, hundreds of you will join me in a live conversation with top medical experts as COVID-19 vaccines begin to roll out around the country. You will get access to people who usually only appear on the networks and big papers. I invite you to record the exchange and use it in your reporting.
You will hear from Dr. Paul Offit, who was on the FDA advisory panel that voted on Thursday; Dr. Susan Bailey, the head of the American Medical Association; Dr. Leon McDougle, the head of the National Medical Association; Patsy Stinchfield, the head of the National Foundation for Infectious Diseases.
We will try to stay narrowly focused on the vaccine and the logistics of the national vaccination effort. We want you to get answers to questions that your viewers, listeners and readers want to know.
If you have not registered, please do so now. I expect around a thousand people to join in.
What studies are showing about young children and COVID-19’s spread

Students walking in the hallway at Tibbals Elementary School place their arms in front as a reminder to socially distance in Murphy, Texas, Thursday, Dec. 3, 2020. (AP Photo/LM Otero)
National Geographic explores a growing body of evidence about how younger children are so different from even older kids when it comes to catching COVID-19 and passing it to others. It is the kind of data that could guide future decisions to open classrooms.
Many studies agree that age matters. One recent preprint tracked 4,524 people from 2,267 houses in Geneva, Switzerland, from April through June. The researchers found that children from 5 to 9 were up to 22.7 percent less likely to be infected, and that their risk increased with age.
The takeaway is that a critical shift appears somewhere between the ages of 10 and 12. Around the time of puberty, the risk of teenagers both getting and transmitting the virus increases. The COVID Monitor, a group tracking information from more than 7,000 U.S. school districts, found that high school case rates are nearly three times that of elementary schools.
It’s still unclear why that might be the case. One theory is that children are more frequently exposed to coronaviruses, conferring some protection. Another is that children have fewer ACE2 receptors, a target of the coronavirus, in their upper airways. Still another theory is that their smaller lungs aren’t as good at projecting droplets or generating aerosols.
Germany heads for Christmas lockdown
We have learned to look at Asia and Europe for cues about what might be next in the pandemic and Germany is about to lock down for Christmas. Starting Wednesday, nonessential stores, schools and hairdressers will be required to close, and companies will be encouraged to offer employees an extended holiday break or allow them to work from home until Jan. 10. The lockdown includes New Year’s Eve celebrations. France, Ireland and England are relaxing their rules.
Electoral College Day
This is usually about the least interesting day on the election calendar, but this year being 2020, we will be watching electors from each state sign their respective documents pledging to support Joe Biden or Donald Trump.
By law, the electors meet the first Monday after the second Wednesday in December. This year it falls on Dec. 14. (The Electoral College is a unique method for indirectly electing the president of the United States. It was established by Article II, Section 1, Clause 2 of the U.S. Constitution and modified by the 12th and 23rd Amendments.)
It is not the most photogenic or exciting thing to cover, but the National Conference of State Legislatures explains this is how it usually works.
The electors meet in each state capitol and cast their ballots for president and vice president. Each elector votes on his or her own ballot and signs it. The ballots are immediately transmitted to various people: one copy goes to the president of the U.S. Senate (who is also the vice president of the United States); this is the copy that will be officially counted later. Other copies go to the state’s secretary of state, the National Archives and Records Administration, and the presiding judge in the district where the electors meet (this serves as a backup copy that would replace the official copy sent to the president of the Senate if it is lost or destroyed).
For reasons that no longer apply, the certified documents must arrive at the U.S. Senate by Dec. 23, nine days later.
There is no federal law or constitutional provision requiring electors to vote for the party that nominated them, and over the years a number of electors have voted against the instructions of the voters. Electors generally are selected by the political party for their party loyalty, and many are party leaders, and thus not likely to vote other than for their party’s candidate. The NCSL says these states have laws that bind electors to the vote:
Most of the laws cited above require electors to vote for the candidate of the party that nominated the elector, or require the elector to sign a pledge to do so. Some go further:
Oklahoma imposes a civil penalty of $1,000; in North Carolina, the fine is $500, the faithless elector is deemed to have resigned, and a replacement is appointed. In South Carolina, an elector who violates his or her pledge is subject to criminal penalties. In New Mexico a violation is a fourth degree felony. In Michigan, a candidate who fails to vote as required is considered to have resigned, and a replacement is appointed.
The times in which we live
And of course, there is @kkariko, whose tireless work on mRNA was critical to these vaccines
A brilliant Hungarian immigrant, she lives outside Philly
Because science is global
There is no “American vaccine”
Thankfully Americans realize that, even if our politicians don’t
— Ashish K. Jha, MD, MPH (@ashishkjha) December 13, 2020
We’ll be back tomorrow with a new edition of Covering COVID-19. Sign up here to get it delivered right to your inbox.